Suppose a reacting flow consists of k = 1, …,
Q species and
j = 1, …,
N reactions. The equation for the first
Q − 1 species is given by
where and Vk is the multicomponent diffusion velocity for species
k. The definition of
Vk depends on the option chosen for the Diffusion Model property. If Mixture Averaged is chosen then:
The mean molar mass, Mn is computed using the sum of the mass fractions and molecular weights of all species:
The mole fraction for species k, which is required in
Equation 5-4 can be computed from the mass fraction of species
k and the mean molar mass:
The quantity, Dkj is the binary diffusion coefficient between species
k and
j and are rather complicated functions of the physical properties of the species. They are defined later, in
Equation 5-13.
where q is the unit charge (SI unit: s A) and
kB is Boltzmann’s constant (SI unit: J/K).
where Dk,f is the user-defined diffusion coefficient for species
k. The mobility for species
k is then given by:
When Dalgarno Ref. 8 is selected in the Mobility Specification section the ion mobility is computed as:
where α is the background atom polarizability in Å
3,
N0 = 2.69
·10
25 m
−3 is the gas number density at 273.15
°C and 760 Torr, and
mr is the reduced mass
Dalgarno’s mobility corresponds to the polarizability limit of the classical Langevin theory Ref. 9. This mobility model gives reasonable values for alkali ions in Ar, Kr, Xe, N
2 and H
2. This model should not be used in the case resonant charge exchange contributes significantly to the ion transport as it occurs in ions drifting in the parent gas. Moreover, Dalgarno’s mobility is only valid for small electric field strengths that ensure that the ion thermal velocity is much larger than the ion drift velocity. Note that the ion mobility and ion drift velocity are, respectively, independent and directly proportional to the electric field.
where σ is a cross section characterizing the ion-atom interaction (SI unit: m
2). This model is valid when the electric field is high enough to ensures that the ion drift velocity is much larger than the ion thermal velocity
Ref. 8. With this model the ion drift velocity is proportional to
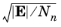
as is frequently found in experiments in the high field limit.
By default, the mixture averaged diffusivity Dk,m (and Fick’s law diffusivity
Dk,f) are computed using
Einstein’s relation:
There is also the option to use the Local Field Approximation. The local field approximation can be used to compute the ion temperature and thus the diffusivity through Einstein’s relation, see
Ref. 7 and
Ref. 10. The ion temperature is computed using:
where Mk is the ion mass (SI unit: kg),
T is the background gas temperature (SI unit: K),
Mn is the mean mass of the mixture (SI unit: kg), and
E is the electric field (SI unit: V/m). If the High field mobility model is used the background gas temperature is neglected and the ion temperature is computed as