You are viewing the documentation for an older COMSOL version. The latest version is
available here.
The Droplet Evaporation feature causes particles to decrease in size over time, by treating them as liquid droplets evaporating in a surrounding gas.
In the physics interface Additional Variables section, you can select one of the following options from the
Particle size distribution list:
Uniform size,
Specify particle diameter, or
Specify particle mass.
|
•
|
If Uniform size is selected, then you control the particle size directly from the Particle Properties settings window, in which you can specify any two of the following three material properties:
|
|
•
|
If either Specify particle diameter or Specify particle mass is selected, then you only specify the Particle density in the settings for the Particle Properties node. Then, in the settings for release features such as Inlet and Release from Grid, you can either set the initial particle diameter or mass directly, or sample it from a distribution. Compared to the Uniform size option, either of these choices will cause the number of degrees of freedom solved for to increase by one per particle, since the particle mass or diameter is now considered a dependent variable to be solved for, rather than a fixed value.
|
Because droplet evaporation models involve model particles whose sizes change over the duration of the study, the Droplet Evaporation node can only be added to the model if either
Specify particle diameter or
Specify particle mass is selected from the
Particle size distribution list. If the
Uniform size option is selected at any point, than any
Droplet Evaporation nodes will be automatically disabled.
Optionally, you can also solve for the particle temperature by selecting the Compute particle temperature check box in the physics interface
Additional Variables section. As described in later sections, the evaporation of a droplet can often be treated as a transient heat-up period followed by a period of evaporation at a fixed temperature. If a complete model of both the heat-up and steady-state periods is desired, then the
Compute particle temperature check box should be selected and the
Stefan-Fuchs evaporation model should be used. This further increases the number of degrees of freedom by one per particle. The initial temperature of the droplets can be set in particle release features such as
Inlet and
Release from Grid.
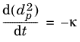
for some constant κ (SI unit: m
2/s). This behavior, which has also been shown experimentally, is sometimes called the
d2 law, and
κ is sometimes called the evaporation constant (
Ref. 32,
35,
39,
40). You can enter the value of
κ directly by selecting
Specify evaporation constant from the
Evaporation model list. This approach can give reasonable approximations of the droplet lifetime if the heat-up time is comparatively small. Typical values of
κ for mixtures of hydrocarbon fuels with air are on the order of 1 mm
2/s (
Ref. 35).
The Maxwell model for droplet evaporation is a quasi-steady model of diffusion of vapor from the surface of a liquid sphere. The diffusion equation in the gas surrounding an isolated droplet is (
Ref. 36)
(5-22)
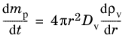
|
•
|
mp (SI unit: kg) is the droplet mass,
|
|
•
|
Dv (SI unit: m 2/s) is the diffusion coefficient of the vapor in the surrounding gas, and
|
|
•
|
ρv (SI unit: kg/m 3) is the mass density of vapor.
|
Spherical symmetry has been assumed, so that the gradient of the vapor density is only nonzero in the radial (r) direction. Thus
Equation 5-22 is only strictly valid when the droplet is perfectly spherical and has zero velocity relative to the surrounding gas.
For r >
rp where
rp is the particle radius,
Equation 5-22 states that the rate of change of the droplet mass is the product of the surface area of a sphere of radius
r with the inward mass flux at
r. The total mass flow rate across any spherical shell with a radius
r >
rp must be equal to that of any other spherical shell, or else mass would be accumulating at some radial distance in the gas, violating the steady-state assumption. Therefore
dmp/dt is a constant, independent of the radial position
r.
In comparing Equation 5-22 to Equation 1.1 in
Ref. 36, the former seems to be missing a negative sign. However,
Ref. 36 defines the evaporation rate, which would be positive for evaporating droplets; whereas
Equation 5-22 defines the time derivative of the particle mass, which would be negative for evaporating droplets.
where the subscript (a) indicates ambient or free-stream conditions a large distance away from the droplet surface, while the subscript
s indicates conditions at the droplet surface. Rearranging this expression gives the so-called Maxwell solution for evaporation of a spherical droplet (
Ref. 34),
(5-23)
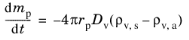
where R = 8.3144598 J/(mol K) is the universal gas constant, gives the Maxwell evaporation model in terms of the partial vapor pressures at the particle surface and in ambient conditions. The vapor pressure at the surface of the droplet is the saturation vapor pressure because the gas surrounding the particle is assumed to be in thermodynamic equilibrium.
(5-24)
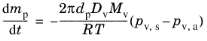
The Stefan-Fuchs evaporation model is an attempt to improve upon the
Maxwell model by including the effect of Stefan flow, the bulk motion of the gas-vapor mixture in the region surrounding the droplet.
Assuming also that the diffusion coefficient of the vapor in the gas equals the diffusion coefficient of the gas in the vapor (Dv = Dg), and also that both phases can be treated as ideal gases, the bulk velocity in the radial direction
vr (SI unit: m/s) is
Equation 5-22 now has both diffusive and advective components,
(5-25)
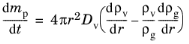
Another simplifying assumption is necessary to solve Equation 5-25. Fuchs (
Ref. 36) suggests that the total molar concentration and total pressure are held constant,
whereas Sazhin (Ref. 34) instead suggests that the total mass density should be held constant,
(5-26)
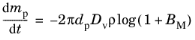
where Mg and
Mv (SI unit: kg/mol) are respectively the molar masses of the ambient gas and vapor phases. Thus the mass transfer number can be determined from the saturation vapor pressure at the droplet temperature, the mass fraction or vapor pressure in the gas at large distances from the droplet, and the molar masses of the vapor and the surrounding gas.
If the saturation vapor pressure is very low, which can be true for relatively cool droplets, then ρg,s and
ρ are nearly equal, and a first-order Taylor expansion of
Equation 5-26 returns the Maxwell solution,
Equation 5-23. For example, Fuchs (
Ref. 36) observed that for water droplets at 20°C, the inclusion of Stefan flow changes the evaporation rate by about one percent.
The Saturation vapor pressure at droplet surface pv,s can be specified directly or solved for using the Clausius-Claperyon equation. One approximate relation that can be used to define
pv,s is
(5-27)
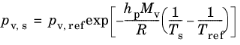
where pv,ref is the saturation vapor pressure at some reference temperature
Tref (SI unit: K) and
hp (SI unit: J/kg) is the droplet latent heat of vaporization. In some scientific literature, slightly different simplifications of the Clausius–Clapeyron equation are presented, or empirical relations with different fitting parameters may be given (
Ref. 32,
34,
35,
36,
38).
An accurate value of the binary diffusion coefficient Dv of the vapor in the surrounding gas is crucial to realistic predictions of droplet lifetimes. In particular, the diffusion coefficient may be highly temperature-dependent.
The Vapor diffusion coefficient may be
User defined, or taken
From thermal properties. The option From thermal properties includes the assumption that the Lewis number is unity,
where ρt is the total density of the vapor-gas mixture. In other words, the mass diffusivity and thermal diffusivity of the surrounding gas are assumed to be equal. In this case, the evaporation rate (following the
Stefan-Fuchs model) becomes (
Ref. 35)
(5-28)
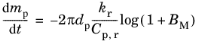
where Cp,r is the specific heat capacity of the vapor-gas mixture, and similarly
kr is the thermal conductivity of the mixture. The rule used to compute these mixture-averaged quantities is to weigh the properties of the vapor and gas phases by their mass fractions,
(5-29)
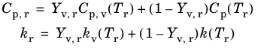
where Yv,r is the reference mass fraction. Here
Cp,v(Tr) means that if the specific heat of the vapor is temperature dependent, then it will be evaluated at the reference temperature
Tr, and similarly for the other material properties shown.
where Tf is the ambient temperature of the surrounding fluid.
If the Compute particle temperature check box is selected in the physics interface
Additional Variables section, then the particle temperature is an additional dependent variable to be solved on each particle, as discussed in the section
Computing Particle Temperature. Recall that the square of the droplet diameter tends to decrease at a constant rate after the droplet reaches some equilibrium temperature called the wet-bulb temperature, at which 100% of the energy transferred to the droplet is used to elicit phase change in molecules at the droplet surface, rather than increasing temperature.
(5-30)
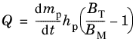
where BT is the dimensionless Spalding heat transfer number,
From Equation 5-30 it is evident that the droplet will heat up if the Spalding heat transfer number is greater than the mass transfer number, or cool down if the Spalding mass transfer number is greater.