
|
|
1
|
|
2
|
In the Application Libraries window, select Corrosion Module>Atmospheric Corrosion>atmospheric_corrosion_busbar_geom in the tree.
|
|
3
|
Click
|
|
1
|
|
2
|
|
3
|
In the tree, select Electrochemistry>Primary and Secondary Current Distribution>Secondary Current Distribution (cd).
|
|
4
|
|
5
|
In the tree, select Electrochemistry>Primary and Secondary Current Distribution>Current Distribution, Shell (cdsh).
|
|
6
|
|
7
|
|
1
|
|
2
|
|
3
|
|
4
|
Browse to the model’s Application Libraries folder and double-click the file atmospheric_corrosion_busbar_parameters.txt.
|
|
1
|
In the Model Builder window, under Component 1 (comp1) right-click Secondary Current Distribution (cd) and choose Electrode.
|
|
2
|
|
3
|
|
1
|
|
2
|
|
3
|
|
1
|
|
2
|
|
3
|
|
4
|
|
1
|
|
2
|
|
3
|
|
4
|
|
1
|
|
2
|
|
3
|
|
4
|
|
5
|
In the tree, select Built-in>Copper.
|
|
6
|
|
1
|
|
2
|
|
3
|
|
1
|
|
2
|
|
3
|
|
1
|
|
2
|
|
3
|
|
4
|
|
5
|
|
6
|
|
1
|
|
2
|
|
1
|
In the Model Builder window, under Component 1 (comp1)>Current Distribution, Shell (cdsh) click Electrolyte 1.
|
|
2
|
|
3
|
|
4
|
|
1
|
|
2
|
|
3
|
|
4
|
|
1
|
|
2
|
|
3
|
|
4
|
Locate the Electrode Kinetics section. From the Kinetics expression type list, choose Anodic Tafel equation.
|
|
5
|
|
6
|
|
1
|
|
2
|
|
3
|
|
4
|
Locate the Electrode Kinetics section. From the Kinetics expression type list, choose Cathodic Tafel equation.
|
|
5
|
|
6
|
|
7
|
|
8
|
|
1
|
|
2
|
|
3
|
|
1
|
|
2
|
|
3
|
|
4
|
|
5
|
|
1
|
|
2
|
|
3
|
|
4
|
|
1
|
In the Model Builder window, under Component 1 (comp1)>Current Distribution, Shell (cdsh) right-click Electrode Surface 2 and choose Duplicate.
|
|
2
|
|
3
|
|
1
|
|
2
|
|
3
|
|
4
|
|
5
|
|
1
|
|
2
|
|
3
|
|
4
|
|
1
|
|
2
|
|
3
|
|
1
|
|
2
|
|
3
|
Find the Studies subsection. In the Select Study tree, select Preset Studies for Selected Physics Interfaces>Stationary with Initialization.
|
|
4
|
|
5
|
|
1
|
|
2
|
|
3
|
|
1
|
|
2
|
In the Settings window for Current Distribution Initialization, locate the Physics and Variables Selection section.
|
|
3
|
|
4
|
|
1
|
|
2
|
In the Settings window for 3D Plot Group, type Electrode Potential vs. Ground (cd) in the Label text field.
|
|
1
|
|
2
|
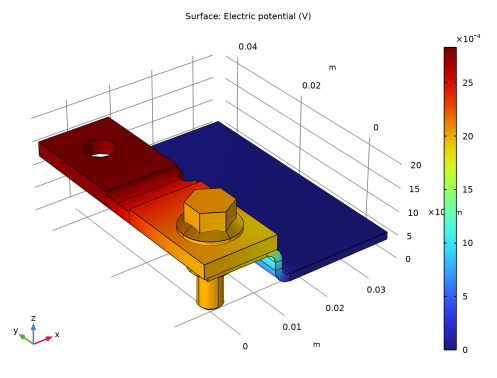
In the Settings window for Surface, click Replace Expression in the upper-right corner of the Expression section. From the menu, choose Component 1 (comp1)>Secondary Current Distribution>cd.phis - Electric potential - V.
|
|
3
|
|
1
|
|
2
|
In the Settings window for 3D Plot Group, type Electrode Potential vs. Adjacent Reference (cdsh) in the Label text field.
|
|
1
|
|
2
|
In the Settings window for Surface, click Replace Expression in the upper-right corner of the Expression section. From the menu, choose Component 1 (comp1)>Current Distribution, Shell>cdsh.Evsref - Electrode potential vs. adjacent reference - V.
|
|
3
|
|
1
|
|
2
|
In the Settings window for 3D Plot Group, type Electrolyte Potential in Film (cdsh) in the Label text field.
|
|
1
|
|
2
|
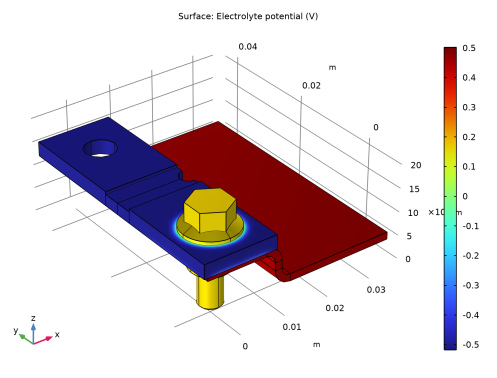
In the Settings window for Surface, click Replace Expression in the upper-right corner of the Expression section. From the menu, choose Component 1 (comp1)>Current Distribution, Shell>phil2 - Electrolyte potential - V.
|
|
3
|
|
1
|
|
2
|
In the Settings window for 3D Plot Group, type Corrosion Current Density (cdsh) in the Label text field.
|
|
1
|
|
2
|
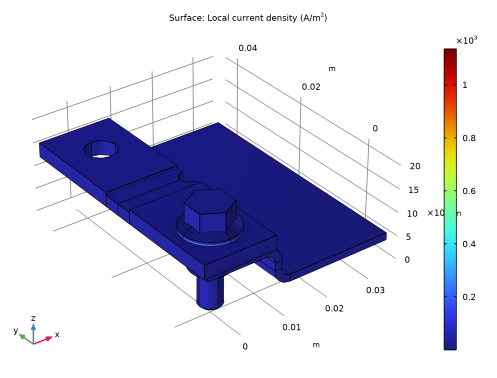
In the Settings window for Surface, click Replace Expression in the upper-right corner of the Expression section. From the menu, choose Component 1 (comp1)>Current Distribution, Shell>Electrode kinetics>cdsh.iloc_er1 - Local current density - A/m².
|
|
3
|
|
1
|
|
2
|
In the Settings window for 3D Plot Group, type Oxygen Reduction Current Density (cdsh) in the Label text field.
|
|
1
|
|
2
|
In the Settings window for Surface, click Replace Expression in the upper-right corner of the Expression section. From the menu, choose Component 1 (comp1)>Current Distribution, Shell>Electrode kinetics>cdsh.iloc_er2 - Local current density - A/m².
|
|
3
|