This phase transformation model is based on the work of Leblond and Devaux (Ref. 1). The model primarily considers carbon-diffusion-based phase transformations that occur in steels during heat treatment. Such transformations include austenite to ferrite, and austenite to bainite. There are three formulations for the Leblond–Devaux model:
where the phase transformation is active only when 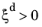 ; that is, when the right-hand side of Equation 3-2
; that is, when the right-hand side of Equation 3-2 is strictly positive. In general, the functions
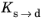
and
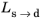
are functions of temperature
T. It was shown in
Ref. 1 that the bainitic transformation additionally depends on the rate of cooling,
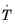
. In this case, the functions
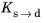
and
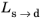
are functions of both
T and
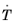
.
where the phase transformation is active only when 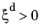 ; that is, when the right side of Equation 3-3
; that is, when the right side of Equation 3-3 is strictly positive. The equilibrium phase fraction
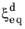
and the time constant
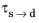
are typically functions of temperature.
In the TTT diagram in Figure 3-1, a curve representing a fixed destination phase fraction
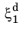
is shown. At a fixed temperature
T, this destination phase fraction is reached at time
t1, so that
where t1 will vary with temperature, and the relative phase fraction
X1 is understood to be the relative phase fraction corresponding to
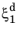
.
where the explicit time dependence has been eliminated. The phase transformation is active only when 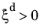 ; that is, when the right side of Equation 3-9
; that is, when the right side of Equation 3-9 is strictly positive. For the special case of
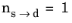
, the equation reduces to the time-and-equilibrium form of the Leblond–Devaux model (
Equation 3-3). The JMAK phase transformation model in
Equation 3-9 has a mathematical disadvantage in that an initial destination phase fraction equal to zero will yield a trivial zero solution, as the logarithm will evaluate to zero. There are different ways to circumvent this problem. One way is to require the initial phase fraction be assigned a small, but finite, value. Another way is to modify the rate equation itself, so that a zero initial phase fraction does not yield a trivial zero solution. In the phase transformation interfaces, the JMAK phase transformation model in
Equation 3-9 is modified for small values on the phase fraction
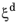
. Below a certain threshold, the argument for the logarithm is modified so that the logarithm does not produce a zero value. This threshold phase fraction is set to 10
-6 by default.
As in the case of the Leblond–Devuax model, the JMAK model can be calibrated using TTT diagram data. The integrated form in Equation 3-8 is used to calibrate the time constant
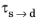
and the Avrami exponent
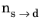
. To calibrate these two phase transformation model parameters, two curves are needed from a TTT diagram, see
Figure 3-2. At a fixed temperature
T, the two destination phase fractions are reached at times
t1 and
t2, respectively, so that
where the relative phase fractions X1 and
X2 are understood to be the relative phase fractions corresponding to
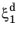
and
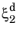
, respectively. The transformation times
t1 and
t2 will vary with temperature.
This phase transformation model is based on the work by Kirkaldy and Venugopalan (Ref. 10), and extended and modified by several others. There are two formulations for the Kirkaldy–Venugopalan phase transformation model:
where the relative phase fraction X has been used. Note that at a fixed temperature, the equilibrium phase fraction
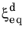
is constant, and it can therefore be included in the rate term
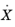
in
Equation 3-16. The reference rate
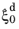
is temperature dependent (and dependent on chemical composition and grain size, in the original Kirkaldy–Venugopalan formulation). At a fixed temperature,
t1 is the time to reach the destination phase fraction
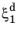
(or alternatively, to reach the relative phase fraction
X1), see
Figure 3-3. This is expressed as
Using Equation 3-16, the rate coefficient is expressed as
Note that if the retardation coefficient Cr is known, the integral can be computed
a priori for a fixed
X1. The rate coefficient is therefore inversely proportional to the time it takes to reach the relative phase fraction
X1.
This phase transformation model was developed by Koistinen and Marburger (Ref. 2) to model the diffusionless (displacive) austenite-martensite transformation in iron-carbon alloys and carbon steels. The onset of the transformation, which only occurs on cooling, is characterized by a critical start temperature — the martensite start temperature
Ms. Above this temperature, no transformation from austenite (the source phase) to martensite (the destination phase) occurs. Below
Ms, the amount of formed martensite is proportional to the undercooling below
Ms, given by
Ms − T. On rate form, the Koistinen–Marburger equation can be written
where β is the Koistinen–Marburger coefficient. Note that the transformation of austenite into martensite only occurs below
Ms and only during cooling (that is, when
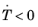
). To make the onset of martensitic transformation numerically smooth, a parameter
ΔMs is used. The smoothing parameter defines a smoothed Heaviside function that makes the onset of martensitic transformation gradual. The parameter should be chosen small enough that the start temperature characteristic is retained. Assuming a constant cooling rate and that the phase fraction of austenite at
Ms is
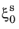
, the rate equation can be integrated to
This integrated form is commonly found in the literature. The rate form of Equation 3-20 is more general, and from a computational standpoint it is more suitable for implementation. The rate form is therefore used in the phase transformation interfaces.