In all the Electrochemistry branch interfaces, the dependent potential variables are 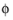 s
s (SI unit: V), the electric potential of the electrode phase (the electron conductor, such as metal), and
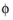 l
l (SI unit: V), the potential of the electrolyte phase (ion conductor).
where Eeq (SI unit: V) is the equilibrium potential. If it is to apply for all overpotentials, a general kinetic expression for an electrode reaction must be set up so that the charge-transfer current over the electrolyte-electrode interface is zero for zero overpotential (equilibrium conditions).
An implication of Equation 4-17 is that it is the potential difference,
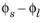
, that governs the kinetics, not the absolute individual values of
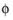 s
s and
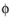 l
l. A global change in the reference for both potentials has no impact on the electrode kinetics. As a result of this, the potentials have to be “boot-strapped” in a model in some way, typically by making an arbitrary choice of electric ground — for example, on an external boundary — in order to ensure that there is a unique solution to the problem.