
|
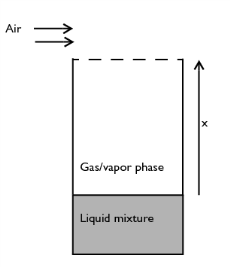
|
1
|
|
2
|
In the Select Physics tree, select Chemical Species Transport>Transport of Concentrated Species (tcs).
|
|
3
|
Click Add.
|
|
4
|
|
5
|
In the Mass fractions table, enter the following settings:
|
|
6
|
Click Study.
|
|
7
|
|
8
|
Click Done.
|
|
1
|
|
2
|
|
3
|
|
4
|
|
1
|
|
2
|
|
3
|
|
4
|
Browse to the model’s Application Libraries folder and double-click the file stefan_tube_parameters.txt.
|
|
1
|
In the Model Builder window, under Component 1 (comp1) click Transport of Concentrated Species (tcs).
|
|
2
|
In the Settings window for Transport of Concentrated Species, locate the Transport Mechanisms section.
|
|
3
|
|
4
|
|
5
|
|
6
|
|
1
|
In the Model Builder window, expand the Transport of Concentrated Species (tcs) node, then click Transport Properties 1.
|
|
2
|
|
3
|
|
4
|
|
5
|
|
6
|
|
7
|
|
8
|
|
9
|
|
1
|
In the Model Builder window, under Component 1 (comp1)>Transport of Concentrated Species (tcs) click Initial Values 1.
|
|
2
|
|
3
|
|
4
|
|
1
|
|
3
|
|
4
|
|
5
|
|
6
|
|
7
|
|
1
|
|
3
|
|
4
|
|
5
|
|
1
|
|
2
|
|
3
|
|
4
|
|
1
|
|
2
|
|
3
|
|
4
|
Browse to the model’s Application Libraries folder and double-click the file stefan_tube_exp.csv.
|
|
1
|
|
2
|
|
1
|
|
2
|
Click in the Graphics window and then press Ctrl+A to select all domains.
|
|
3
|
|
4
|
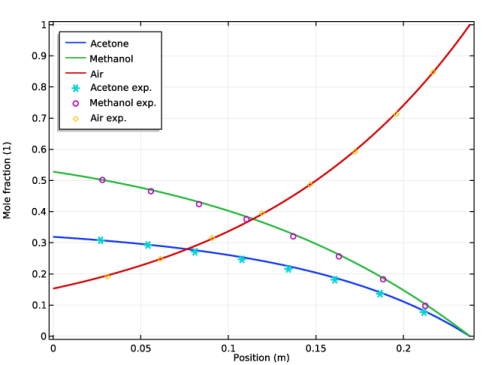
Click Replace Expression in the upper-right corner of the y-axis data section. From the menu, choose Model>Component 1>Transport of Concentrated Species>tcs.x_w1 - Mole fraction.
|
|
5
|
|
6
|
|
7
|
|
8
|
|
9
|
Click to expand the Coloring and style section. Locate the Coloring and Style section. In the Width text field, type 2.
|
|
10
|
|
11
|
|
1
|
|
2
|
In the Settings window for Line Graph, click Replace Expression in the upper-right corner of the y-axis data section. From the menu, choose Model>Component 1>Transport of Concentrated Species>tcs.x_w2 - Mole fraction.
|
|
3
|
Locate the Legends section. In the table, enter the following settings:
|
|
1
|
|
2
|
In the Settings window for Line Graph, click Replace Expression in the upper-right corner of the y-axis data section. From the menu, choose Model>Component 1>Transport of Concentrated Species>tcs.x_w3 - Mole fraction.
|
|
3
|
Locate the Legends section. In the table, enter the following settings:
|
|
1
|
|
2
|
|
3
|
|
4
|
Locate the Coloring and Style section. Find the Line markers subsection. From the Marker list, choose Cycle.
|
|
5
|
|
6
|
|
7
|
|
1
|
|
2
|
|
3
|
|
4
|
In the associated text field, type Position (m).
|
|
5
|
|
1
|
|
2
|
|
3
|
|
4
|
In the associated text field, type Position (m).
|
|
1
|
|
2
|
Click in the Graphics window and then press Ctrl+A to select all domains.
|
|
3
|
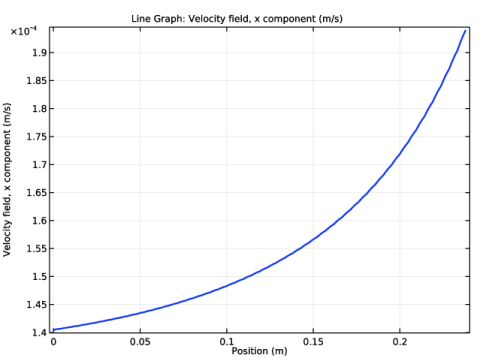
In the Settings window for Line Graph, click Replace Expression in the upper-right corner of the y-axis data section. From the menu, choose Model>Component 1>Transport of Concentrated Species>Velocity field>tcs.u - Velocity field, x component.
|
|
4
|
|
5
|
|
6
|
Click to expand the Coloring and style section. Locate the Coloring and Style section. In the Width text field, type 2.
|
|
7
|