The electrochemical potential μi (J/mol) of the individual species are defined as
where R is the molar gas constant (8.31 J/mol/K),
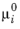
is the electrochemical potential with respect to some reference state,
T is the temperature (K),
xi is the molar fraction, the
λi the species activity coefficient (dimensionless) and
Φ (V) is the solution potential.
where the derivative should be made keeping the temperature, pressure and all electrolyte components but the ones with index j and
k constant. In the Concentrated Electrolyte Transport interface, the index
k equals the index of the electrolyte component taken from the molar fraction constraint.
where Dij (m
2/s) are the binary Maxwell–Stefan diffusivity coefficients. The above equation system is transformed and inverted in order express the electrolyte component fluxes in terms of the gradients of the electrolyte component chemical potentials.