The Blackbody Radiation node releases rays diffusely from a surface (following Lambert’s cosine law) with a wavelength or frequency distribution following Planck’s law (if release of polychromatic rays has been enabled) and a total source power based on the Stefan–Boltzmann law (if ray power is solved for).
An ideal blackbody (Ref. 7) is an object that absorbs all radiation that is incident upon it, regardless of the body’s temperature or the wavelength of the radiation.
Real-world objects usually do not behave as ideal blackbody radiation sources. The spectral emissivity ε(
λ,
T) of an object (dimensionless) is the radiant energy flux per unit surface area the object releases, divided by the radiant energy flux per unit surface area that an ideal blackbody at the same temperature would release. By definition,
ε = 1 for an ideal blackbody.
The total radiant exitance 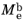 (SI unit: W/m2
(SI unit: W/m2) is the amount of electromagnetic energy emitted from the surface of the blackbody radiation source per unit time, per unit surface area.
The energy released from a blackbody source follows a wavelength distribution. For some value of the wavelength λ, imagine a sensor that detects 100% of the radiation having wavelengths between
λ and
λ +
dλ, where
dλ is an infinitesimally small change in the wavelength, but detects 0% of radiation outside this range. Then the amount of energy detected by this sensor is
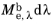
, where
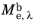
is the spectral radiant exitance. The units of spectral radiant exitance are energy per unit time per unit surface area, per unit wavelength.
The most concise way to write the unit is W/m3 but sometimes it is more conveniently expressed with the area and wavelength units written separately, for example, W/(cm
2·
μm). The spectral radiant exitance is usually a function of wavelength and temperature, so it may be written as
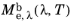
.
For any two wavelengths λ1 and
λ2 (assume
λ1 <
λ2), the total energy of emitted radiation between these wavelengths is
The total energy between two wavelengths λ1 and
λ2 should be the same when converting these wavelengths to frequencies
ν1 =
c/
λ1 and
ν2 =
c/
λ2,
The term cos θ appears in the denominator because the radiance is measured per unit projected surface area from the point of view of an observer at polar angle
θ, rather than the actual surface area from which the radiation is emitted.
The spectral radiance in terms of wavelength 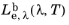 has SI units of W/(m3
has SI units of W/(m3·sr), or it may be written with the area units and wavelength units separated, for example W/(cm
2·nm·sr). Similarly the spectral radiance in terms of frequency
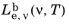
has SI units of (W·s)/(m
2·sr) but can also be written using different area units.
In general, the spectral radiance may be a function of the polar angle θ. However, the ideal blackbody radiation source is assumed to be a Lambertian source, that is, a diffuse emitter of radiation, meaning the direction distribution of released radiation follows the cosine law. This cosine term exactly cancels out the cosine term appearing in the denominator of
Equation 3-8. Therefore the spectral emittance of an ideal blackbody radiation source is independent of the viewing angle.
Although any differential area on the surface of the blackbody releases radiation into the hemispherical half-space above it (subtending a solid angle of 2π steradians), the conversion between exitance and radiance only requires a multiplication by
π rather than 2
π. This has been shown in the above derivation to follow from the treatment of an ideal blackbody as a Lambertian source.
One might be inclined to substitute λ =
c/
ν into
Equation 3-9 or substitute
ν =
c/
λ into
Equation 3-10. Doing so would give seemingly contradictory expressions for the spectral exitance, but doing so would be a mistake because the exitance is not energy flux itself but rather a distribution function of the energy flux (
Ref. 7). A better comparison is to integrate
Equation 3-9 and
Equation 3-10 over matching wavelength and frequency ranges, respectively, noting from
Equation 3-7 that these integrals should agree.
where σ is the Stefan–Boltzmann constant,
In the COMSOL implementation, when polychromatic radiation is permitted in a model (by selecting either Polychromatic, specify vacuum wavelength or
Polychromatic, specify frequency from the physics interface
Wavelength distribution of released rays list), values of
x are first sampled from the above probability distribution function and then converted to the vacuum wavelength or frequency as needed.