Using the Reaction Engineering or
Chemistry interfaces, all species property parameters and property functions required by these interfaces can be created automatically by coupling to an existing
Thermodynamic System,
Predefined System, or
External Thermodynamic System under
Thermodynamics. Examples of species properties that can be created automatically are molar mass, heat capacity, enthalpy, and entropy for each species. Parameters and functions for these properties are created by the thermodynamic system. The
Reaction Engineering and
Chemistry interfaces can also be used to define transport properties for the resulting mixture (all species in the interface). When coupled, the following mixture properties can be automatically created: heat capacity, density, thermal conductivity, and dynamic viscosity.
Note that using a thermodynamic system significantly increases the modeling capabilities in the Reaction Engineering and
Chemistry interfaces. All ideal and nonideal thermodynamic models, for gases and liquids, are directly available and also automatically updated by editing the settings for the thermodynamic system in use. The
Chemistry interface can furthermore be used to make the mixture properties readily available in space-dependent models for modeling of mass transport, heat transfer, or fluid flow.
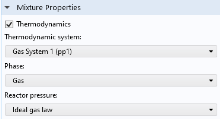
Select a thermodynamic system from the Thermodynamic system list. Use the
Phase list to select the phase to be used in the
Reaction Engineering interface.
The Species Matching section is activated when the
Thermodynamics check box is selected in the
Mixture Properties section; see above. The species in the Reaction Engineering interface can be matched to a species in the thermodynamic system. This step ensures that the arguments in the thermodynamic system functions are correctly defined.
Use the lists in the From Thermodynamics column to match each species in the interface to a species in the coupled thermodynamic system.
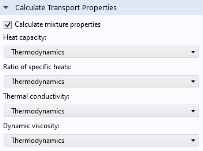
When the interface is fully coupled to a thermodynamic system, property functions for the mixture transport properties are created automatically when the Calculate mixture properties check box is selected. The properties calculated by the thermodynamic system display
Thermodynamics in the corresponding list; see below.
You can couple a Chemistry interface with an existing thermodynamic system in the Chemistry interface’s settings window. You can make this coupling in the Settings window for the
Chemistry interface by selecting the
Thermodynamics check box in the
Mixture Properties section. You need to have at least one species defined in the Chemistry interface in order to couple it to a thermodynamic system.
Select a thermodynamic system from the Thermodynamic system list. Use the
Phase list to select the phase to be used in the
Chemistry interface.
The Species Matching section is activated when the
Thermodynamics check box is selected in the
Mixture Properties section. Here you can match the variables for the concentrations, and by this calculate mixture properties (transport and thermodynamic properties). For information on how to specify the dependent variables to be used, see
Species Matching in
The Chemistry Interface documentation in the
Chemical Reaction Engineering Module User’s Guide.
You can match the species in the Chemistry interface with those in the corresponding thermodynamic system in the Species matching section’s table. Use the lists in the column with the title
From Thermodynamics to match each species in the Chemistry interface to a species in the corresponding thermodynamic system. This ensures that the composition function arguments in the thermodynamic system are correctly defined.
When all species in a physics interface (Reaction Engineering or
Chemistry) are matched to the corresponding species in a thermodynamic system, the mixture properties are calculated based on the composition of the mixture. For example, consider
Zmix(
T,
P,
n1, …,
nm), which denotes a generic extensive mixture property for a mixture of
m number of species. The property function’s arguments are the temperature
T, the pressure
P, and the number of moles,
n, for each species.

