The Equilibrium Calculation functionality is used to compute the resulting equilibrium conditions for a mixture of a set of species and phases.
The Equilibrium Calculation Wizard consists of the following steps:
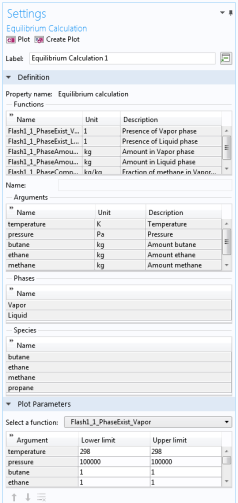
The Equilibrium Calculation settings include three types of equilibrium functions.
Select one or more of the species available in the thermodynamic system and use the Add Selected button (

) to add them to the
Selected species table. Click the
Next button (

) to proceed to the next step in the wizard.
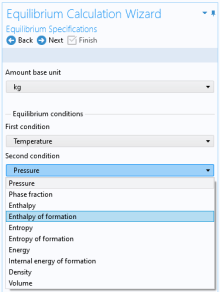
Select two Equilibrium conditions that define the current equilibrium, for example a given pressure and a temperature. These equilibrium conditions are used as arguments in the equilibrium functions, in addition to the composition (overall fractions of species).
The available equilibrium conditions are: Temperature,
Pressure,
Phase fraction,
Energy (or
Internal energy of formation),
Enthalpy (or
Enthalpy of formation),
Specific volume,
Density, and
Entropy (or
Entropy of formation). For chemical reactions, it is recommended to use
Enthalpy of formation,
Entropy of formation, or
Internal energy of formation, since they account for heat of reactions.
Selecting Phase fraction as one of the equilibrium conditions activates the
Solution type input field. This can be used to indicate the direction of the desired solution, which is of great use especially near critical points. The options
Undefined,
Normal, or
Retrograde define different directions for the search of the solution to the equilibrium equations. Using
Normal means that the derivative of the vapor phase fraction with respect to temperature (at constant pressure and composition) is kept positive and the derivative of the vapor phase fraction with respect to pressure (at constant temperature and composition) is kept negative. Using
Retrograde means that the opposite sign of the previous mentioned derivatives are enforced.
Note that the Solution type setting is only available for a built-in thermodynamic systems. For external thermodynamic systems, the corresponding functionality needs to be supplied by the thermodynamic software provider. For instance, the COCO/TEA provider does not support the
Normal or
Retrograde options. In those cases, the
Solution type should be
Undefined.
Click the Next button (

) to proceed to the next step.
In this step, you can review all the functions including units and arguments. Click the Finish button (

) to exit the wizard and add functions at equilibrium state to the current thermodynamic system.
When creating an Equilibrium Calculation, the resulting functions are collected under the
Mixture node. You can create new functions from an existing
Mixture node. Right-click it and select
Equilibrium Calculation to start the
Equilibrium Calculation Wizard (see
Figure 2-20).
Selecting an Equilibrium Calculation node displays the settings including the property functions, see
Figure 2-22.