The apparent heat capacity formulation provides an implicit capturing of the phase change interface, by solving for both phases a single heat transfer equation with effective material properties. The latent heat of phase change is taken into account by modifying the heat capacity.
The Phase Change Material domain condition should be used on both phases domains, to solve the heat equation after specifying the properties of the phase change material according to the
apparent heat capacity formulation.
Instead of adding a latent heat L in the energy balance equation exactly when the material reaches its phase change temperature
Tpc, it is assumed that the transformation occurs in a temperature interval
ΔT. Within this interval, the material is transformed from phase 1 to phase 2. The corresponding volume fractions of the phases are represented by the phase transition function
α 1 → 2, where
θ1 = 1 − α 1 → 2 is the volume fraction of the material before transition and
θ2 = α 1 → 2 the volume fraction of the material after transition. See the section
Phase Transition Function for details about the form of
α 1 → 2.
The density, ρ, and the specific enthalpy,
H, are expressed by:
Finally, the apparent heat capacity, Cp, used in the heat equation, is given by:
The apparent heat capacity, Cp, used in the heat equation, is given by:
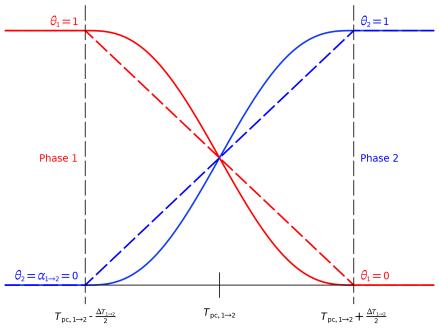
How the phase transition from phase 1 to phase 2 takes place is described by the phase transition function α 1 → 2. By default, COMSOL uses a smoothed step function with a continuous second derivative (Heaviside function). It describes a continuous transition from phase 1 to phase 2 within the transition interval
ΔT around the phase transition temperature T
pc. Besides the frequently used Heaviside function, a linear function is also common and available as predefined option.
Figure 4-2 illustrates the functions and the corresponding parameters.
With a user-defined phase transition function, the volume fraction of phase 2 is explicitly specified. For reasonable results, the value range of α 1 → 2 should be between 0 and 1 satisfying
θ1 + θ2 = 1 and the function should be differentiable to correctly approximate the latent heat contribution, which is calculated from the derivative of this function by
Equation 4-49. A user-defined function can also be used to describe a phase transition where not the complete volume fraction of one phase changes into the other, that is, there is a residual phase.
