For an example of how to use the Packed Bed, see the model example A Multiscale 3D Packed Bed Reactor, file path Chemical_Reaction_Engineering_Module/Reactors_with_Porous_Catalysts/packed_bed_reactor_3d
|
For an example of how to use the Packed Bed, see the model example A Multiscale 3D Packed Bed Reactor, file path Chemical_Reaction_Engineering_Module/Reactors_with_Porous_Catalysts/packed_bed_reactor_3d
|
|
•
|
εb is the bed porosity.
|
|
•
|
The dependent variable c for each chemical species i represents the interparticle concentration, that is, the concentration based on unit volume of fluid flowing between the pellets.
|
|
•
|
Ri is the species interparticle reaction rate in the bed. This corresponds to reactions occurring in the macropores between the pellets in the bed. Note that Ri is the reaction rate per unit volume of bed.
|
|
•
|
εpe is the pellet (microscale) porosity.
|
|
•
|
cpe,i is the intraparticle concentration of species i in moles/m3 of fluid volume inside the micropores.
|
|
•
|
Rpe,i is the pellet reaction rate. This corresponds to reactions taking place inside the pellets. Note that the user input of Rpe,i is per unit volume of pellet.
|
|
•
|
Continuous concentration: assuming that all resistance to mass transfer to/from the pellet is within the pellet and no resistance to pellet-fluid mass transfer is on the bulk fluid side. The concentration in the fluid will thus be equal to that in the pellet pore just at the pellet surface:
|
|
•
|
Film resistance (mass flux): The flux of mass across the pellet-fluid interface into the pellet is possibly rate determined on the bulk fluid side by film resistance. The resistance is expressed in terms of a film mass transfer coefficient, hDi, such that:
|
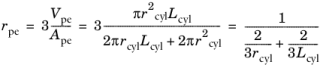 .
.|
•
|
rc is the pellet core radius (m)
|
|
•
|
|
•
|
Msolid is the solid species molar mass
|
|
•
|
ρpe is the pellet core density
|