In presence of chemical reactions, the enthalpy (SI unit: J/kg) is a function of temperature, pressure and the mixture composition. For a mixture of N components, the mass fractions verify the condition
ω1 + …
+ ωN = 1. Therefore the enthalpy is a function of the temperature, pressure, and only
N − 1 mass fractions:
where Qvd is the heat source from viscous dissipation, and
Q represents additional sources. The heat flux
q consists in a term of conduction and a term of diffusion:
Introducing the heat capacity at constant pressure Cp (SI unit: J/(kg·m
3), and the coefficient of thermal expansion
αp (SI unit: 1/K) gives:
The term with the pressure derivative is the work of pressure forces Qp. The enthalpy variation due to the gradient of species mass flux can be partly simplified with the diffusion part of the heat flux. The heat source of reaction can be written in terms of enthalpies of reaction
Hi instead of partial enthalpies (see
Ref. 9). The temperature derivative term can be expanded to make the convective term appear. We finally obtain the temperature equation for a free flow with
N species and
M reactions:
where εp is the porosity of the medium (SI unit: 1),
Sarea is the specific surface area (SI unit: 1/m), and
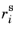
is the surface reaction rate (SI unit: mol/(m
2·s)).
This equation is the one that is solved when the Porous medium type of the
Porous Medium feature is set to
Local thermal nonequilibrium, since both fluid and porous matrix temperatures are solved separately. With the
Local thermal equilibrium approach, the equation is written for the common temperature, and in terms of effective material properties for the time derivative term and conduction term: