where νki are the stoichiometric coefficients and
ck is the concentration of the
kth species. The surface rate expression (SI unit: mol/(m
2·s)) for species
k is defined as
where νki = νrki − νfki. The rate of progress of the
ith reaction is governed by the law of mass action:
where wk is the mass fraction of species
k,
ρ is the gas density, and
Mk is the molecular weight of species
k. When the Motz-Wise correction option is set to On, the forward rate constant is related to the sticking coefficients via:
where Γtot is the total surface site concentration (SI unit: mol/m
2). The
sticking coefficient can be a constant or even temperature dependent through an Arrhenius expression:
where a,
b, and
e are constants. Since
γ is a probability it must be between 0 and 1 to be physically meaningful.
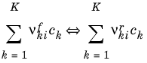
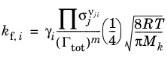 .
.