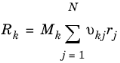
|
•
|
|
•
|
kf,j is the rate coefficient for the jth forward reaction (SI unit: 1/s for first order reactions, m3/(mol·s) for second order reactions or m6/(mol2·s) for third order reactions)
|
|
•
|
|
•
|