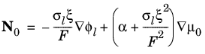A complication when modeling the water-ion-polymer system is that the water-ion friction forces need to be taken into account in the transport equations both for the ions as well as the water molecules. The Weber-Newman model (Ref. 7) is based on concentrated solution theory, taking into account all three binary interactions between the charge-carrying ion, the immobilized polymer matrix and the water molecules. The model introduces the chemical potential of water in the polymer
μ0 (J/mol) as dependent variable, and defines the polymer water flux,
N0 (mol/(m
2·s)), as
where ξ (dimensionless) is the electroosmotic coefficient and
α (mol
2/(J·m·s)) the water transport coefficient, with the mass balance
where a0 is the thermodynamic activity of water,
VH2O is the partial molar volume of water, and
pH2O(l) is the liquid water pressure.
VH2O is defined as
where MH2O is the molar mass and
ρH2O is the density of water at the operating temperature.
where μ0,ref is a chosen reference potential, for convenience set to 0.
For a vapor-equilibrated membrane, the parameters σl,
ξ, and
α typically all depend on the polymer water content. When measuring water uptake and other polymer electrolyte properties (such as
σl,
ξ, and
α), it is common to report these versus the water activity,
aw, which is not the same as the thermodynamic activity
a0.
where is the pH2O(g) is the partial water pressure,
pvap is the vapor pressure, and
pg is the gas pressure. At an interface between a polymer electrolyte and the gas phase, assuming the polymer phase to be in equilibrium with the gas phase (and ideal gas conditions), the following condition holds
For a membrane in contact with vapor, the liquid pressure pH2O(l) is 0 Pa, and the chemical potential
μ0 relates to the thermodynamic activity
a0 as
where kabs,dsp (mol/(m
2 s)) is a rate constant and the activity of water in the gas phase has been assumed to equal the partial pressure
pH2O(g) divided by the standard condition pressure
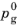
(ideal gas conditions).
For a membrane in contact with liquid water, aw is equal to 1. Interfaces of this kind have been empirically found to exhibit way lower mass transfer resistances than for the vapor case. For this case, the boundary condition may be formulated based on the liquid pressure
pH2O(l) as
For this cases the absorption rate rabs,dsp may be evaluated at the boundary as
The boundary heat source Qb due to the absorption-desorption is defined as