where T denotes the temperature (K),
Mi the molecular weight of species
i (g/mol
),
P is the pressure
(Pa), and
vi are the atomic diffusion volumes (Fuller diffusion volume)
(cm
3).
where ηi,v is the vapor viscosity of each individual species, and
Mi are the molecular weights.
In the above formula, the summations are done for all active species, where λi,v and
ηi,v are the individual vapor thermal conductivity and dynamic viscosity correlations as a functions of temperature for the pure gases, respectively. The normal boiling points
Tb,i and the species molecular weights are also considered. The factor
Sij equals 0.773 if either
i or
j represents the index of water (steam). For all other cases
Sij =1.
where Cp,mix is the heat capacity of the fluid mixture in J/(kg·K),
cp,i is the species heat capacity in J/(mol·K).
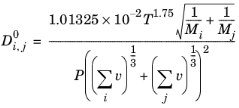
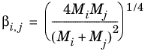 ,
,