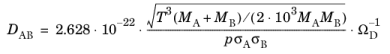The Reaction Engineering Interface and
The Chemistry Interface can calculate several transport properties that can be accessed in interfaces in space dependent models.
Here, DAB (SI unit: m
2/s) is the binary diffusion coefficient,
M (SI unit: kg/mol) equals the molecular weight,
T (SI unit: K) represents the temperature,
p (SI unit: Pa) is the pressure, and
σ (SI unit: m) equals the characteristic length of the Lennard-Jones/ Stockmayer potential. In addition,
ΩD is the collision integral, given by the relation of Neufeld et al. (
Ref. 6):
In Equation 2-62,
cx are empirical constants,
μD is the species dipole moment value (SI unit: Cm) and
ε/
kb (SI unit: K) the potential energy minimum value divided by Boltzmann’s constant. Tabulated data in literature frequently lists values of
ε/
kb rather than
ε. It should be noted that predefined expressions for binary diffusivities only treat ideal gas mixtures. Thus, these are applicable as input only for gases at moderate pressure in multicomponent diffusive transport models.
The binary diffusivity according to Equation 2-61 is also suited for gaseous species in solvent, simply by setting either the component A or B to the solvent. The binary diffusion coefficient is in this case equal to the diffusion coefficient of the bulk species.
The diffusivity of a species A in a solvent
B is calculated with the Wilke–Chang equation (
Ref. 8):
where μB (SI unit: N·s/m
2) denotes the solvent’s dynamic viscosity (see
Equation 2-69), and
Vb,A (SI unit: m
3/mol) equals the molar volume at the normal boiling point of the solute species.
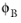
is the dimensionless association factor of the solvent, which by default is set to 1.
In Equation 2-65, μ (SI unit: Ns/m
2) represents the dynamic viscosity, and
ΩD is the dimensionless collision integral given by:
In Equation 2-65 and
Equation 2-66,
bx are empirical constants,
μD (SI unit: Cm) the species dipole moment value, and
ε/
kb (SI unit: K) the potential energy minimum value divided by Boltzmann’s constant. Tabulated data in literature frequently lists values of
ε/
kb rather than
ε, and
σ (SI unit: Å) is the characteristic length value.
In Equation 2-67 and
Equation 2-68, xi is the molar composition and
μi is computed with
Equation 2-65 for each of the species in the mixture.
where μ (SI unit: Ns/m
2) is the dynamic viscosity. As inputs for
Equation 2-69, the physics interface takes the reference viscosity,
μref (SI unit: Pa
⋅s) at the reference temperature
Tref (SI unit: K).
where k (SI unit: W/(m·K)) is the thermal conductivity and
Cp (SI unit: J/(mol·K)) denotes the molar heat capacity.
Equation 2-70 is a function of viscosity,
μ, as given by
Equation 2-65.
Equation 2-70 is directly used in the case of a solvent; all parameters being those of the solvent. Without a solvent, however, the following equation is also used:
where ki is the thermal conductivity of each species
i and
xi the molar composition for each of the species in the mixture.