where νi is the stoichiometric coefficient of the reacting species of index
i and
n is the number of participating electrons.
The equilibrium potential of the electrode reaction, Eeq (V), is the electrode potential (the difference between the electrode phase and electrolyte phase potentials,
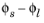
) for which the net reaction rate (and the local current density,
iloc) is zero.
where Eeq, ref (V) is the equilibrium potential for a reference state for which all species activities
ai (unitless) are equal to a chosen set of reference activities
ai, ref (unitless).