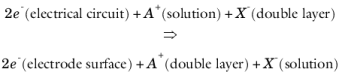This corresponds to a negative charge of 2F being moved from double layer to solution. Following the convention that the reaction is written in the order of cathodic current (as above), then with respect to the free solution species, the cation is a reactant (negative stoichiometry) and the anion is a product (positive stoichiometry). This suggests
νA =
−1,
νX =
+1, and
n = 2 for an ideal double layer where both anion and cation have similar contributions to the double layer charge.