
|
|
•
|
|
•
|
|
•
|
|
1
|
|
2
|
|
3
|
Click Add.
|
|
4
|
Click
|
|
5
|
In the Select Study tree, select Preset Studies for Selected Physics Interfaces>Time Dependent with Initialization.
|
|
6
|
Click
|
|
1
|
|
2
|
|
3
|
|
4
|
Browse to the model’s Application Libraries folder and double-click the file li_battery_multiple_materials_parameters.txt.
|
|
1
|
|
2
|
|
3
|
|
5
|
|
1
|
|
2
|
|
3
|
|
4
|
|
5
|
|
6
|
|
7
|
|
8
|
|
9
|
|
10
|
|
11
|
|
1
|
In the Model Builder window, expand the Component 1 (comp1)>Materials>NCA, LiNi0.8Co0.15Al0.05O2 (Positive, Li-ion Battery) (mat3)>Equilibrium potential (elpot) node, then click Interpolation 1 (Eeq_int1).
|
|
2
|
|
1
|
In the Model Builder window, expand the Component 1 (comp1)>Materials>LMO, LiMn2O4 Spinel (Positive, Li-ion Battery) (mat4)>Equilibrium potential (elpot) node, then click Interpolation 1 (Eeq_int1).
|
|
2
|
|
1
|
|
2
|
|
3
|
|
4
|
Browse to the model’s Application Libraries folder and double-click the file li_battery_multiple_materials_variables.txt.
|
|
1
|
In the Model Builder window, under Component 1 (comp1) right-click Lithium-Ion Battery (liion) and choose Porous Electrode.
|
|
3
|
|
4
|
|
5
|
|
6
|
|
1
|
|
2
|
|
3
|
|
4
|
Locate the Particle Transport Properties section. From the Species concentration transport model list, choose Baker-Verbrugge.
|
|
5
|
|
6
|
|
7
|
|
1
|
|
2
|
|
3
|
|
4
|
|
1
|
|
3
|
|
4
|
From the Electrode material list, choose NCA, LiNi0.8Co0.15Al0.05O2 (Positive, Li-ion Battery) (mat3).
|
|
5
|
|
6
|
|
7
|
Locate the Effective Transport Parameter Correction section. From the Electrolyte conductivity list, choose User defined. In the fl text field, type epsl_pos^brugl_pos.
|
|
8
|
|
1
|
|
2
|
|
3
|
From the Particle material list, choose NCA, LiNi0.8Co0.15Al0.05O2 (Positive, Li-ion Battery) (mat3).
|
|
4
|
|
5
|
|
1
|
|
2
|
|
3
|
|
4
|
|
1
|
|
3
|
In the Settings window for Additional Porous Electrode Material, locate the Volume Fraction section.
|
|
4
|
|
1
|
|
2
|
|
3
|
|
4
|
|
5
|
|
6
|
Locate the Model Input section. From the c list, choose Solid phase concentration, Additional Porous Electrode Material 1 (liion/addm1/pin1).
|
|
1
|
|
2
|
|
3
|
|
4
|
|
5
|
Locate the Model Input section. From the c list, choose Insertion particle surface concentration, Additional Porous Electrode Material 1 (liion).
|
|
1
|
|
3
|
|
4
|
|
5
|
Locate the Effective Transport Parameter Correction section. From the Electrolyte conductivity list, choose User defined. In the fl text field, type epsl_sep^brugl_sep.
|
|
6
|
|
1
|
|
2
|
In the Settings window for Initial Cell Charge Distribution, locate the Battery Cell Parameters section.
|
|
3
|
|
4
|
|
5
|
|
1
|
In the Model Builder window, expand the Initial Cell Charge Distribution 1 node, then click Negative Electrode Selection 1.
|
|
1
|
|
1
|
|
1
|
|
3
|
|
4
|
|
1
|
|
2
|
|
3
|
|
4
|
|
1
|
|
2
|
|
3
|
|
4
|
|
1
|
|
2
|
|
3
|
|
1
|
|
2
|
|
3
|
|
1
|
|
2
|
|
3
|
|
4
|
|
5
|
Right-click Study 1>Solver Configurations>Solution 1 (sol1)>Time-Dependent Solver 1 and choose Stop Condition.
|
|
6
|
|
7
|
Click
|
|
9
|
|
10
|
|
11
|
|
12
|
|
13
|
|
1
|
|
2
|
|
3
|
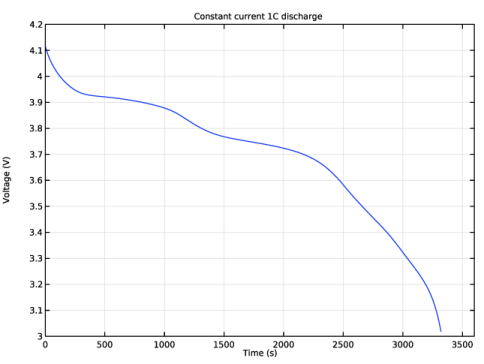
In the Rename 1D Plot Group dialog box, type Constant current 1C discharge in the New label text field.
|
|
4
|
Click OK.
|
|
1
|
|
3
|
In the Settings window for Point Graph, click Replace Expression in the upper-right corner of the y-Axis Data section. From the menu, choose Component 1 (comp1)>Lithium-Ion Battery>phis - Electric potential - V.
|
|
1
|
|
2
|
|
3
|
|
4
|
In the associated text field, type Voltage (V).
|
|
5
|
|
6
|
|
7
|
|
8
|
|
9
|
|
10
|
|
11
|
|
1
|
|
2
|
|
3
|
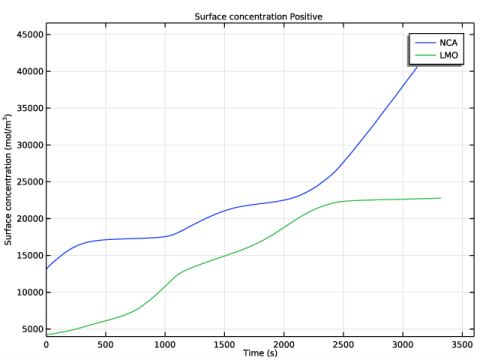
In the Rename 1D Plot Group dialog box, type Surface concentration Positive in the New label text field.
|
|
4
|
Click OK.
|
|
1
|
|
3
|
In the Settings window for Point Graph, click Replace Expression in the upper-right corner of the y-Axis Data section. From the menu, choose Component 1 (comp1)>Lithium-Ion Battery>Particle intercalation>liion.cs_surface - Insertion particle concentration, surface - mol/m³.
|
|
4
|
|
5
|
|
1
|
|
2
|
In the Settings window for Point Graph, click Replace Expression in the upper-right corner of the y-Axis Data section. From the menu, choose Component 1 (comp1)>Lithium-Ion Battery>Particle intercalation>liion.cs_surface_addm1 - Insertion particle surface concentration, Additional Porous Electrode Material 1 - mol/m³.
|
|
3
|
Locate the Legends section. In the table, enter the following settings:
|
|
1
|
|
2
|
|
3
|
|
4
|
In the associated text field, type Surface concentration (mol/m<sup>3</sup>).
|
|
5
|
|
6
|
|
7
|
|
8
|
|
9
|
|
10
|
|
1
|
|
2
|
|
3
|
In the Settings window for Solution, type Study 1/Solution 1: xdim Negative in the Label text field.
|
|
4
|
Locate the Solution section. From the Component list, choose Extra Dimension from Particle Intercalation 1 (liion_pce1_pin1_xdim).
|
|
1
|
|
2
|
|
3
|
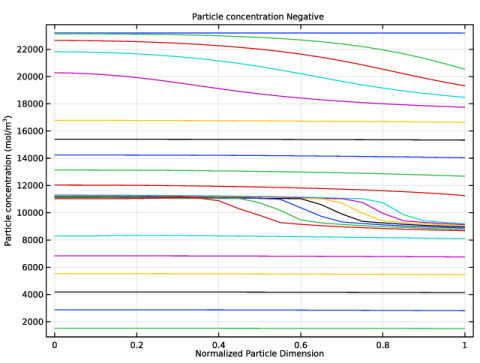
In the Rename 1D Plot Group dialog box, type Particle concentration Negative in the New label text field.
|
|
4
|
Click OK.
|
|
5
|
|
6
|
|
7
|
|
8
|
|
1
|
|
3
|
|
4
|
|
1
|
|
2
|
|
3
|
|
4
|
In the associated text field, type Normalized Particle Dimension.
|
|
5
|
|
6
|
In the associated text field, type Particle concentration (mol/m<sup>3</sup>).
|
|
7
|
|
8
|
|
1
|
|
2
|
|
3
|
Find the Studies subsection. In the Select Study tree, select Preset Studies for Selected Physics Interfaces>Time Dependent with Initialization.
|
|
4
|
|
5
|
|
6
|
|
1
|
|
2
|
|
1
|
|
2
|
|
3
|
Click
|
|
1
|
|
2
|
|
3
|
|
4
|
|
5
|
|
6
|
Right-click Study 2>Solver Configurations>Solution 3 (sol3)>Time-Dependent Solver 1 and choose Stop Condition.
|
|
7
|
|
8
|
Click
|
|
10
|
|
11
|
|
12
|
|
13
|
|
14
|
|
1
|
|
2
|
|
3
|
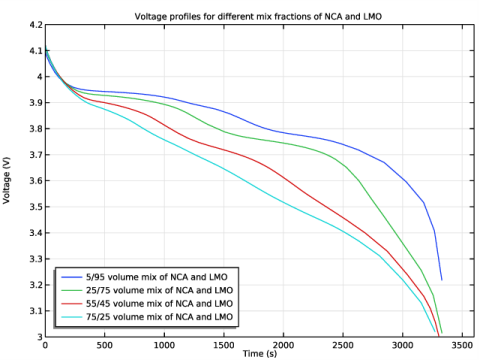
In the Rename 1D Plot Group dialog box, type Voltage profiles (parametric) in the New label text field.
|
|
4
|
Click OK.
|
|
1
|
|
2
|
|
3
|
|
5
|
Click Replace Expression in the upper-right corner of the y-Axis Data section. From the menu, choose phis - Electric potential - V.
|
|
6
|
|
7
|
|
8
|
In the Legend text field, type eval(fr_pos_NCA*100)/eval((1-fr_pos_NCA)*100) volume mix of NCA and LMO.
|
|
1
|
|
2
|
|
3
|
|
4
|
In the associated text field, type Voltage (V).
|
|
5
|
|
6
|
|
7
|
|
8
|
|
9
|
|
10
|
|
11
|
|
12
|
|
13
|