|
•
|
|
•
|
|
•
|
|
•
|
|
•
|
pv is the partial pressure of water vapor
|
|
•
|
p is the pressure
|
|
•
|
|
•
|
Moisture content xvap.
|
|
•
|
Vapor mass fraction omega_moist.
|
|
•
|
Relative humidity phi. This variable corresponds to the calculated φ with the system temperature and pressure.
|
|
•
|
Saturation indicator satInd; this indicator is set to 1 if saturation has been detected (
|
|
•
|
ht.feature.fc(RH,T, pA), where RH is the relative humidity
|
|
•
|
ht.feature.fxvap(RH, T, pA), where RH is the relative humidity
|
|
•
|
ht.feature.fpsat(T), where T is the temperature (SI unit: K). It returns the saturation pressure (SI unit: Pa) by using Equation 4-29.
|
|
•
|
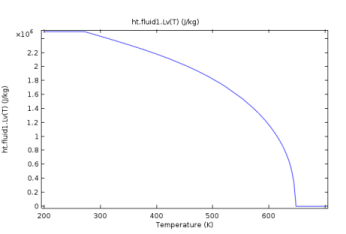
ht.feature.Lv(T), where T is the temperature (SI unit: K). It returns the latent heat of evaporation (SI unit: J/kg) as a linear interpolation of the data from Ref. 39, which provides steam properties based on the Industrial Formulation IAPWS-IF97. The temperature-dependency is as shown on Figure 4-1.
 |