You are viewing the documentation for an older COMSOL version. The latest version is
available here.
The Reaction Engineering (re) interface (

), found under the
Chemical Species Transport branch (

) when adding a physics interface, is used to model several chemical reactor types and the evolution of chemical reactions over time. The mass balance and energy balance equations describing these systems assume perfect or well-defined mixing in the reactor. Essentially, the physics interface simulates, tank, and plug flow chemical reactors to investigate the behavior over time of a chemical reaction.
The reaction kinetics expressions of a reactor can be exported to a space-dependent model, using the Generate Space-Dependent Model feature. This gives you the power to simulate reacting systems as they depend on fluid flow, mass transfer, and heat transfer — in other words, including space dependencies.
Add physics features from the toolbar, or right-click Reaction Engineering to select features from the context menu. Many of the fields and nodes described in this section are made available when either a
Reaction or a
Species (or both) subnode is added to the Model Builder. Because nodes and subnodes are accessible at any time, and any change is updated throughout the model, reactions and species are often defined before the settings described in this section.
The Label is the default physics interface name.
The Name is used primarily as a scope prefix for variables defined by the physics interface. Refer to such physics interface variables in expressions using the pattern
<name>.<variable_name>. In order to distinguish between variables belonging to different physics interfaces, the
name string must be unique. Only letters, numbers, and underscores (_) are permitted in the
Name field. The first character must be a letter.
The default Name (for the first physics interface in the model setup) is
re.
Select a Reactor type to define the reaction system. The available reactor types are:
Batch,
Batch, constant volume,
CSTR, constant volume,
CSTR, constant mass/generic,
Semibatch, and
Plug flow. Each reactor type solves a mass balance based on properties typical to the type, such as reactor volume, volumetric production, or flow rate:
|
•
|
Batch: In reactors no mass enters or leaves the system. This type can account for a variable reactor volume.
|
|
•
|
Batch, Constant Volume: Same as Batch but where the reactor volume is assumed to be constant. As this is the situation for most reacting systems, this is the default condition.
|
|
•
|
CSTR, Constant Mass/Generic: Continuous stirred tank reactors (CSTR) differ from batch reactors, since these allow species to enter and leave the reactor by means of feed inlet streams and outlet streams. The system’s volume is allowed to change, such as in a car engine cylinder or a balloon. This reactor model can be solved either for a constant mass condition or by selecting a specific outlet flow.
|
|
•
|
CSTR, Constant Volume: Same as the CSTR with constant mass/generic reactor but assumes that the volume is constant during operation.
|
|
•
|
Semibatch: Semibatch reactors differ from batch reactors in that they allow reactants to enter the reactor by means of one or several feed inlet streams.
|
|
•
|
Plug Flow: In the plug flow reactor, the species concentrations and the temperature vary with position. Plug flow in a tubular configuration means that concentration and temperature gradients develop only in the axial direction, but not in the radial direction.
|
For each Reactor type, additional settings are shown in
Table 2-1. If a surface reaction or species is included, except for the
Plug flow reactor type, the surface reaction area is also a parameter. The parameters and expressions are used in the mass balance equations.
For CSTR, constant volume and
Semibatch reactor types, the
Volumetric production rate (
vp) is available. For
Automatic, the in-built
Volumetric production rate expression is shown. If
User defined is selected, the expression can be changed (SI unit: m
3/s). For instance, this enables the setting of zero (0) volumetric production rate, which ignores volume changes due to reactions.
(2-76)
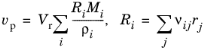
The physics interface automatically inserts the stoichiometric coefficients (νij) and reaction rate expressions for each species (
Ri) that depend on
j number of reactions of a rate (
rj), as defined in the
Reaction feature node. Furthermore, the values of the molar mass (
Mi) and the species density (
ρi) are automatically taken from the
Species features.
(2-77)
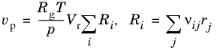
For CSTR, constant mass/generic, select
Volumetric rate to either
Constant mass (Automatic) or
Generic. The
Constant mass (Automatic) selection shows both the expression for
Volumetric production rate (
vp) (
Equation 2-77 for gas, or
Equation 2-76 for liquid phase reactions) and
Volumetric outlet rate (
v) (
Equation 2-78). The latter is derived from constant mass flow condition through the reactor:
(2-78)
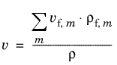
The mixture density (ρf) of
m number of feed inlet streams is determined in the same way as in the
Mixture Properties section. For
Generic both the
Volumetric rate properties can be edited (SI unit: m
3/s). This means that it is possible to completely control the volumetric outlet rate from the CSTR.
For the Plug flow reactor, the
Volumetric flow rate along the reactor is set. The
Automatic definition computes a variable volumetric flow rate that depends on the molar flow rate of each species (
Fi).
(2-79)
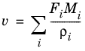
(2-80)
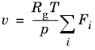 .
.The default value for p (
Reactor pressure) is 1 atm in
Equation 2-81 and it is set in the
Mixture Properties section.
This input field sets the Reactor volume Vr — that is, the fluid volume in which chemical reaction takes place. The
Batch reactor type can account for a changing volume, thus a time-dependent volume expression can be entered here.
Once a surface reaction, or a surface species, has been added, for all reactor types except Plug flow, the
Surface reaction area settings become visible. Here, the area of the surface on which the surface reactions take place can be defined. The surface area can either be defined directly as a parameter, or by defining the
Surface area to volume ratio.
From the list select to Exclude or
Include the energy balance, which in essence determines whether the system is solved for either isothermal or nonisothermal conditions, respectively. The latter introduces the temperature as a variable in the interface.
If Exclude is chosen, enter a
Temperature T for the system.
It is possible to incorporate cooling or heating of the reactor. This is done in External heating or cooling (
Qext). Enter a negative value to account for cooling or a positive one for heating (SI unit: W). Note that for the Plug Flow reactor type, the
External heating or cooling (
Q) is defined per reactor volume (SI unit: W/m
3).
The Thermodynamics check box is enabled except if:
|
•
|
the Thermodynamics node, including one or more systems, is not added under Global Definitions, or
|
Use the Phase list to specify the state of aggregation of the mixture.
This setting is available when the Calculate mixture properties check box in the
Calculate Transport Properties section has been selected.
Two settings are always available for the mixture Density:
Automatic or
User defined. The
Thermodynamics options is available when the interface is coupled to a
Thermodynamic System, and all interface species has been matched to species in the system. In this case the density is defined by a function automatically added under the thermodynamic system coupled to.
The Automatic setting uses the following logic:
|
•
|
Automatic selected for Liquid, considers the liquid as ideal and incompressible. The liquid mixture density depends on the density of i number of pure species ( ρi) and the species mass fraction ( wi).
|
|
•
|
Automatic set for Gas calculates the gas mixture density ( ρ) from the concentrations (c i) and molar masses ( Mi) of the mixture species, which are automatically taken from Species features.
|
(2-81)
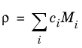
|
•
|
If a Species Type is set to Solvent and the Mixture is Liquid, the mixture density is the same as the solvent density as defined in Density in General parameters in the Species node. When Mixture is Gas, the mixture density is calculated from Equation 2-81 only for the species set as Solvent.
|
The Phase -
Gas setting displays the
Reactor pressure. For all reactor types, except the
Plug flow reactor, select either a pressure computed from the
Ideal gas law or from any other expression using the
User defined option. The
Batch and
Semibatch reactor types also have the option to keep the reactor pressure
Constant during reaction.
For the Plug Flow reactor, the
Reactor pressure can be entered in the case of the
Reactor section having the
Volumetric rate set to
Automatic, in which case the
User defined alternative is available and a constant pressure fits the conditions.
When the Thermodynamics check box is selected in the
Mixture Properties section and the species are fully coupled (see the section below), the reactor pressure is set to
Thermodynamics indicating that it is automatically computed.
The Species Matching section is activated when the
Thermodynamics check box is selected in the
Mixture Properties section. The species in the Reaction Engineering interface can be matched to species in the
Thermodynamic System. This ensures that the arguments in the thermodynamic system functions are correctly defined.
Use the drop-down lists in the From Thermodynamics column to match each species in the interface to a species in the coupled thermodynamic system.
Select the Calculate mixture properties check box to enable calculation of mixture transport properties exported from the Reaction Engineering interface.
From the list for each property, select the in-built Automatic expression or set a
User defined entry. The mixture properties you can transfer for space-dependent models are:
|
•
|
Heat capacity ( cp) (SI unit: J/(kg·K)) (this is available when the Energy Balance is set to Include)
|
|
•
|
Thermal conductivity ( k) (SI unit: W/(m·K))
|
|
•
|
Dynamic viscosity ( μ) (SI unit: Pa·s) (this is available when a Species Type is set to Solvent)
|
|
•
|
Mixture density ( ρ) (SI unit: kg/m 3) (this is available in the Mixture Properties section)
|
This section is available when at least one equilibrium reaction has been defined, that is, when a Reaction node incorporates at least one
Equilibrium reaction (
Reaction type is set to
Equilibrium), or when at least one equilibrium reaction has been defined in an
Equilibrium Reaction Group.
In the Predefined dependent species (separated by ‘,’) text field, edit, if necessary, the species that depends on the composition of the other species according to the
Equilibrium expression in the
Reaction node. To minimize the impact of any numerical errors, it is recommended to set the species with the highest concentration as dependent species. The default species is set to the leftmost species in the
Reaction formula.
The Suppress negative concentrations check-box exists to aid the computation of equilibrium reaction systems. A selected check-box ensures that no negative values of concentrations are accepted as solution to the equilibrium condition.
Select the Use activity check box to solve for species activities instead of species concentrations, which is a common approach when non-ideal fluids are modeled.
This section enables CHEMKIN® import to simulate complex chemical reactions in gas phase.
Two types of CHEMKIN input files can be imported here — Thermo and
Transport, for thermodynamic properties and transport properties respectively. Properties for either volumetric or surface species are supported. Click
Browse to locate the CHEMKIN file to be imported, then click
Import. For Thermo the imported data is directly entered in the
NASA format fields in the
Species Thermodynamic Expressions section. For Transport the imported data is entered in the
Species Transport Expressions section.
To display this section, click the Show More Options button (

) and select
Advanced Physics Options.
The Uniform scaling of concentration variables check box is not selected by default. When selected, all concentration variables are scaled using the same scale factor in the
Study node. Enabling uniform scaling can decrease solver time for problems involving many concentration variables.
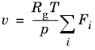 .
.