|
•
|
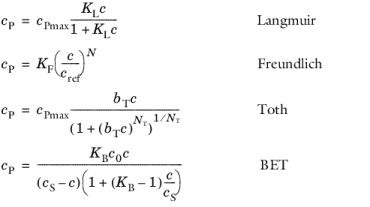
Freundlich: Freundlich constant KF (SI unit:·mol/kg), Freundlich exponent NF (dimensionless), and reference concentration cref (SI unit: mol/m3).
|
|
•
|
|
•
|
Toth: Toth constant bT (SI unit: m3/mol), Toth exponent NT (dimensionless), and adsorption maximum cPmax (SI unit: mol/kg).
|
|
•
|
BET (Braunauer-Emmett-Teller): BET constant KB (dimensionless), and a monolayer adsorption capacity c0 (SI unit: mol/kg) and a Saturation concentration, cS(SI unit: mol/m3).
|