where il denotes the current density vector. In free electrolyte, there is no source or sink of charge.
where Av denotes the specific surface area (dimension
L2/
L3), and
iloc the local current density defines the rate of the charge transfer reactions, for instance according to the Butler-Volmer equation. For various ways of defining
iloc see
Electrode Kinetics Expressions.
where iloc,m (A/m
2) is the current density of the charge transfer electrode reaction of index
m,
il the current density vector in the electrolyte and
is the current density vector in the electrode.
where Av is the specific surface area of the electrocatalyst.
where is denotes the current density vector according to:
and where σs denotes the electrical conductivity and
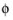 s
s the potential of the electron conducting (metal) phase.
where il, bnd is a given expression for the current density vector.
The feature adds one unknown variable, the electrolyte potential, 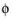 l, bnd
l, bnd, along the boundary. It then adds one additional equation for the total current, which is an integral over the boundary:
The adds one unknown variable, the electric potential,
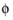 s, bnd
s, bnd, along the boundary. It then adds one additional equation for the total current, which is an integral over the boundary:
and σs denotes the electrode conductivity and
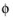 s
s the electric potential. The average current density condition imposes the same equation but multiplies the current density by the area of the boundary to obtain the value of the total current,
In,s.
where ik denotes the current density vector and
k =
l,
s is an index for the electrolyte and electrode, respectively.
and σs denotes the electrode conductivity and
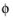 s
s the electric potential.
where is, bnd is a given expression for the current density vector.
where 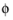 s, ground
s, ground is the ground potential of the cell, and
Ptotal (W) is the power to be drawn.
where Pavg is the average power density on the boundary, and
A is the boundary area.
The electrochemical potential μi of a charged species of index
i is
where T(K) is the temperature,
R (mol/(J K)) the molar gas constant,
ai is the species activity,
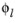
is the electrolyte potential,
zi the species charge, and
F(C/mol) is Faraday's constant.