where U is the internal energy,
P is pressure,
V is volume,
T is temperature,
S is entropy and
H is enthalpy of the system. For a closed system
where δQ is (reversible) heat transfer to the fluid and
δW is (pressure) work in the system. The change in Gibbs free energy can be written as
Chemical potential μi is defined as partial molar Gibbs energy for species
where n is the number of mole of species
i in the system. At constant temperature, this expression can be integrated as a function of pressure
where v is molar volume and
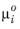
is chemical potential of species at standard state. For an ideal gas this can be expressed as
where νi is the stoichiometric coefficient and
μi is the chemical potential of species
i in the reaction. We can evaluate the chemical potential as partial molar Gibbs energy of species in mixture as
where 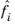 is the fugacity of species i
is the fugacity of species i in the mixture, and
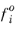
is the fugacity of pure species in the standard state.
Equation 2-12 can be rewritten as
where ai is the activity of species in the system.
Activity depends on the choice of an arbitrary standard state. The standard state of pure species is usually at 105 Pa and for solute in solution is based on hypothetical molality or amount concentration also referred as infinite dilute behavior.
where γi is the activity coefficient of species in the mixture and
fi is the fugacity of pure species at the equilibrium temperature and pressure. The activity is expressed by
where fio is the fugacity of pure species at the equilibrium temperature and 1 atm. The ratio
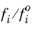
is given by
where 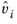 is the partial molar volume of species and Psat
is the partial molar volume of species and Psat is species saturated vapor pressure. For liquids is weak function of pressure and can be assumed to be 1 unless at high pressure.
Additionally, an effective species concentration (ce,i) (SI unit: mol/m
3) is used in the reaction rates (
Equation 2-2) when activities are utilized.