The Lithium-Ion Battery Interface defines the current balance in the electrolyte, the current balances in the electrodes, the mass balance for the lithium salt, and the mass balance of lithium in lithium-ion batteries.
In the electrolyte and pore electrolyte, two variables are defined, 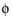 l
l and
cl. Assuming electroneutrality,
cl denotes both the
Li+ concentration and the
An- concentration.
where σl denotes the electrolyte conductivity,
f the activity coefficient for the salt,
t+ the transport number for
Li+ (also called transference number),
itot the sum of all electrochemical current sources, and
Ql denotes an arbitrary electrolyte current source. In the mass balance for the salt,
εl denotes the electrolyte volume fraction,
Dl the electrolyte salt diffusivity, and
Rl the total
Li+ source term in the electrolyte.
where σs is the electrical conductivity.
where Qs is an arbitrary current source term.
where Θs denotes a free reaction site and
LiΘs an occupied reaction site at the solid particle surface.
The concentration of Θs does not have to be solved for since the total concentration of reaction sites,
cs,max, is assumed to be constant, implying that
The equilibrium potentials E0 of lithium insertion electrode reactions are typically functions of
soc.
where cs is the concentration of Li in the solid phase. This equation is solved locally by this physics interface in a 1D pseudo dimension, with the solid phase concentrations at the nodal points for the element discretization of the particle as the independent variables. The gradient is calculated in Cartesian, cylindrical, or spherical coordinates, depending on if the particles are assumed to be best described as flakes, rods or spheres, respectively.
where RLiΘ denotes the molar flux of lithium at the particle surface, caused by the electrochemical insertion reactions.
where the stoichiometric coefficients, νi, is positive (
νox) for products and negative (
νred) for reactants in a reduction reaction. From this definition, the number of electrons,
n, in the electrode reaction can be calculated according to
where zi denotes the charge of species
i. According to these relations, the lithium insertion reaction has the following stoichiometric coefficients:
with a resulting n = 1. These are the default settings for the reactions in this physics interface. When modeling other reactions, such as irreversible anion oxidation or noninsertion solid lithium metal deposition, other coefficients have to be used.
In the porous electrodes, itot, denotes the sum of all charge transfer current density contributions according to:
where, Av denotes the specific surface. The source term in the mass balance is calculated from:
It is also possible to specify additional reaction sources, Rl, src, that contribute to the total species source according to:
where the last factor (normally equal to 1) is a scaling factor accounting for differences between the surface area (Av,m) used to calculate the volumetric current density, and the surface area of the particles in the solid lithium diffusion model.
Nshape is 1 for Cartesian, 2 for cylindrical, and 3 for spherical coordinates.
The molar source RvΘ at the positive and negative electrodes is given as follows:
where Rfilm (SI unit:
Ω·m
2) denotes a generalized film resistance. The activation overpotentials,
ηm, for all electrode reactions in the electrode then receives an extra potential contribution, which yields
where εs denotes the electrode volume fraction and
cs,avg,cycl,electrode is the local average cyclable species concentration defined as:
cs,avg is the average species concentration, which initially, when no concentration gradients are present within the electrode particles, is equal to the concentration at the surface of the electrode particles,
cs,surf.
socmin is the minimum allowed state-of-charge in the electrode material. The amount of cyclable species charge of additional electrode materials is calculated similarly.
where Qhost (SI unit: C) is the amount of active host material,
fcycl,loss the fractional loss of cyclable species, and
fhost,neg,ex the fractional excess of negative active host material.
where Δsoc is the allowed state-of-charge window of the electrode material.
The relative change in volume δV/V0 is typically dependent on the solid phase concentration
cs (or the state-of-charge variable
soc). Note that
cs is solved for in a 1D extra dimension using spherical or cylindrical coordinate systems (for spheres or cylinders, respectively), as described above. In the equations presented below, the relative volume change is considered to be a generic function of the concentration
ΔV/V0 = fvol(cs(r)).
The relationships between stress, σ(r) (SI unit: Pa), and strain,
ε(r) (SI unit: 1), expressed in the spherical coordinate system for the radial and tangential components (considering that
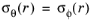
) are
where E (SI unit: Pa) is Young’s modulus and
ν (SI unit:1) is Poisson’s ratio. It is assumed that these elastic properties are independent of concentration.
The expressions for radial and tangential stresses in a spherical particle of radius rp that satisfy the boundary condition
σr(rp) = 0 and remain finite at
r = 0, can be obtained as follows, by solving the equation for static mechanical equilibrium in the absence of any body force:
The hydrostatic stress σh(r) (SI unit: Pa) (or the mean stress) is given by
The strain energy density Ws(r) (SI unit: J/m
3) accumulated as a result of the elastic deformation for the isotropically deformed sphere is given as
The total elastic strain energy density stored in the host electrode material Ws,tot(r) (SI unit: J/m
3), which provides the driving force for fracture, is obtained as,
where εs is the electrode volume fraction in the host material.
The relationships between stress, σ(r), and strain,
ε(r), expressed in the cylindrical coordinate system for the radial, tangential and axial components are as follows:
The strain energy density Ws(r) accumulated as a result of the elastic deformation for the isotropically deformed cylinder is given as