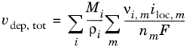If the reaction rate is known, the total growth vdep, tot (m/s) is defined as the sum of the velocity contributions for all species and electrode reactions according to:
Where Mi (SI unit: kg/mol) is the molar mass and
ρi (SI unit: kg/m
3) the density of the species. (
i is the species index, and
m the index of the electrode reaction).
In a time-dependent simulation one may also introduce a surface concentration variable, cs,i (mol/m
2) on the boundary and calculate the accumulated surface concentration change by using a local ordinary differential equation (ODE):
The total deposited thickness stot(m) can then be defined as