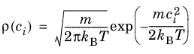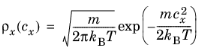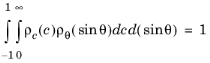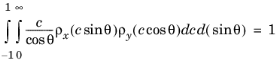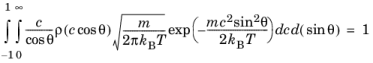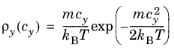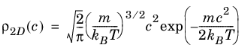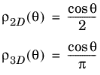For the Free Molecular Flow interface when the Knudsen number is much greater than 1, collisions between gas molecules as they traverse the interior of a system can be ignored. There are two common approaches to solving the flow in this case: the Monte Carlo method (which computes the trajectories of large numbers of randomized particles through the system) and the
angular coefficient method. COMSOL Multiphysics uses the angular coefficient method, which computes the molecular flow by summing the flux arriving at a surface from all other surfaces in its line of sight. The macroscopic variables in the vicinity of the surface can be derived from kinetic theory.
In many cases it is reasonable to assume that molecules are adsorbed and subsequently diffusely emitted from the surface (this is often referred to as total accommodation). In this instance, the appropriate probability density functions for emission from a surface ρ (
θ, c) (SI unit: m
−1s), where
θ is the angle to the normal (SI unit: rad) and
c is the particle speed (SI unit: ms
−1), have been derived from the laws of physical gas dynamics and verified by molecular dynamics simulations (
Ref. 1). A complete derivation of the probability distribution function in 2D is given in this section.
for θ in the range
(−π/2, π/2) for both cylindrical (2D) and polar (3D) coordinates. In 3D the azimuthal angle
φ is in the range
(0, π).
Let cx and
cy be the velocity components in the parallel and wall normal directions, respectively. The relationships between the velocity components in Cartesian and polar coordinates are
Applying Equation 3-13 to the normalization condition yields
Substituting Equation 3-12 into the modified normalization condition then yields
Combining Equation 3-12 and
Equation 3-14 yields the probability distribution function of the speed
c,
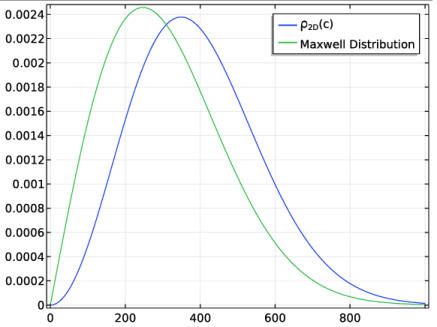
Repeating the derivation of the previous section in 3D yields an analogous probability distribution function; as in 2D, the result is found to differ from the distribution function of c that would be obtained naively from the Maxwell-Boltzmann distribution. The results in both 2D and 3D are summarized below. The polar angle distribution always follows the cosine law,