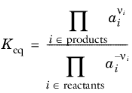
The equilibrium condition is commonly based on the stoichiometric coefficients, νi (dimensionless), of the reaction; the species activities of the reacting species
ai (dimensionless); and an equilibrium constant,
Keq (1) according to:
where ca0 (SI unit: mol/m
3) is the standard molarity, and
γc,i (dimensionless) an activity coefficient.
The Equilibrium Reaction node solves for a reaction rate so that the equilibrium condition is always fulfilled in the domain. It is available for the Modules Chemical Engineering, Corrosion, Electrochemistry, Electrodeposition, and Batteries and Fuel Cells.