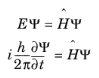The Schrödinger equation (from the Austrian physicist Erwin Schrödinger) is an equation that describes the behavior of the quantum state of a physical system as it changes in time:
where Ψ is the quantum mechanical wave function (the probability amplitude for different configurations of the system) and
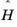
is the
Hamiltonian.
h, the Planck constant, over 2
π is often called the
reduced Planck constant (
h-bar).