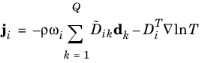
|
•
|
|
|
•
|
T (SI unit: K) is the temperature
|
|
•
|
|
•
|
c (SI unit: mol/m3) is the total molar concentration
|
|
•
|
Rg is the universal gas constant 8.314 J/(mol·K)
|
|
•
|
p (SI unit: Pa) is the total pressure
|
|
•
|
pk (SI unit: Pa) is the partial pressure, and
|
|
•
|
|
•
|
gk (SI unit: m/s2) is an external force (per unit mass) acting on species k. In the case of an ionic species the external force arises due to the electric field.
|