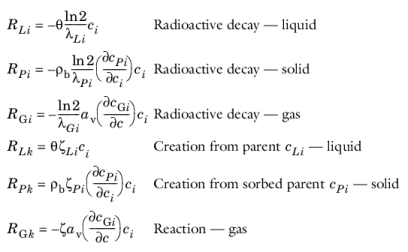
where λ is the chemical half life,
ζ is a reaction rate, and the subscripts
L,
P, and
G denote liquid, solid, and gas phases, respectively. In the equations, the reactions either depend on liquid concentration
ci or solid phase concentrations
cPi obtained using the sorption derivative with
ci or gas phase concentration
cGi depending on the gas volume fraction, the volatilization, and the liquid concentration.
In Equation 3-19,
T denotes the current absolute temperature,
TR denotes the reference absolute temperature,
Ea is the activation energy, and
Ru is the universal gas constant.