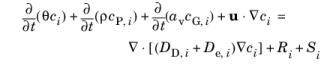The following equations for the molar concentrations, ci, describe the transport of solutes in a variably saturated porous medium for the most general case, when the pore space is primarily filled with liquid but also contain pockets or immobile gas:
On the left-hand side of Equation 3-12, the first three terms correspond to the accumulation of species within the liquid, solid, and gas phases, while the last term describes the convection due to the velocity field
u (SI unit: m/s).
In Equation 3-12 ci denotes the concentration of species
i in the liquid (SI unit: mol/m
3),
cP, i the amount adsorbed to solid particles (moles per unit dry weight of the solid), and
cG, i the concentration of species
i in the gas phase.
The equation balances the mass transport throughout the porous medium using the porosity εp, the liquid volume fraction
θ; the matrix (drained) density,
ρ = (1
− εp)
ρp, and the solid phase density
ρp.
For saturated porous media, the liquid volume fraction θ is equal to the porosity
εp, but for partially saturated porous media, they are related by the saturation
s as
θ =
sεp. The resulting gas volume fraction is
av =
εp − θ =
(1-s)εp.
On the right-hand side of Equation 3-12, the first term introduces the spreading of species due to mechanical mixing resulting from the porous media (dispersion), as well as from diffusion and volatilization to the gas phase. The dispersion tensor is denoted
DD (SI unit: m
2/s) and the effective diffusion by
De (SI unit: m
2/s).
The last two terms on the right-hand side of Equation 3-12 describe production or consumption of the species;
Ri is a reaction rate expression which can account for reactions in the liquid, solid, or gas phase, and S
i is an arbitrary source term, for example due to a fluid flow source or sink.
The time evolution of the adsorption, the solute transport to or from the solid phase, is defined by assuming that the amount of solute adsorbed to the solid, cP,i, is a function of the concentration in the fluid
ci. This implies that the solute concentration in the liquid and solid phase are in instant equilibrium. The adsorption term can be expanded to give
where kP,i = ∂cP,i/∂ci is the adsorption isotherm.
where kG,i = ∂cG,i/∂ci is the linear volatilization.