|
•
|
|
•
|
|
•
|
|
•
|
|
•
|
pv is the partial pressure of water vapor
|
|
•
|
p is the pressure
|
|
•
|
φ is the relative humidity, and
|
|
•
|
|
•
|
Moisture content xvap.
|
|
•
|
Vapor mass fraction omega_moist.
|
|
•
|
Relative humidity phi. This variable corresponds to the calculated φ with the system temperature and pressure.
|
|
•
|
Condensation indicator condInd; this indicator is set to 1 if condensation has been detected (φ = 1) and 0 if not.
|
|
•
|
ht.feature.fc(RH,T, pA), where RH is the relative humidity 0 ≤ φ ≤ 1, T is the temperature (SI unit: K), and pA is the pressure (SI unit: Pa). It returns the corresponding water vapor concentration (SI unit: mol/m3) by deriving the following relation from Equation 4-107, Equation 4-110, and Equation 4-114:
|
|
•
|
ht.feature.fxvap(RH, T, pA), where RH is the relative humidity 0 ≤ φ ≤ 1, T is the temperature (SI unit: K) and pA is the pressure (SI unit Pa). It returns the moisture content (SI unit: 1) by using the following relation:
|
|
•
|
ht.feature.fpsat(T), where T is the temperature (SI unit: K). It returns the saturation pressure (SI unit: Pa) by using Equation 4-111.
|
|
•
|
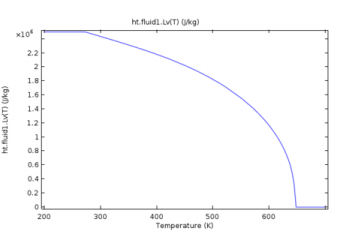
ht.feature.Lv(T), where T is the temperature (SI unit: K). It returns the latent heat of evaporation (SI unit: J/kg) as a linear interpolation of the data from Ref. 36, which provides steam properties based on the Industrial Formulation IAPWS-IF97. The temperature-dependency is as shown on Figure 4-14.
 |