In experimental electrochemistry, it is common to use a reference electrodes when controlling current or voltage with a potentiostat. Potential differences in the system are recorded with respect to the equilibrium potential of the redox couple at the reference electrode. A good reference electrode is designed so that no net charge transfer takes place at its electrode-electrolyte interface. Then the overpotential of the reference is zero, so:
where 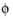 s , ref
s , ref (SI unit: V) is the electric potential of the reference electrode and
Eeq, ref (SI unit: V) is the equilibrium potential of the reference electrode reaction.
Where Evs ref (SI unit: V) is the electrode potential versus the defined the electric reference potential.