Using a Fick’s law approximation, the relative mass flux due to molecular diffusion is assumed to be governed by
where 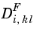 represents a general diffusion matrix (SI unit: m2
represents a general diffusion matrix (SI unit: m2/s) describing the diffusion of species
i into the mixture. This form makes it possible to use any diffusion coefficient, matrix, or empirical model based on Fick’s law. For example, in situations when the mass transport is not dominated by diffusion, an alternative is to use the diffusion coefficients at infinite dilution,