where ρi is the density and
xi the mole fraction of species
i. The diffusion hence depends on a single concentration gradient and is proportional to a diffusion coefficient
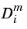
. The diffusion coefficient describes the diffusion of species
i relative to the remaining mixture and is referred to as the mixture-averaged diffusion coefficient.
Equation 3-46 can be expressed in terms of mass fractions as
The mixture-averaged diffusivities are explicitly given by be the multicomponent Maxwell-Stefan diffusivities Dik. As a consequence, no matrix inversion operation is required as for the Maxwell-Stefan diffusion model (when using four or more species). For low-density gas mixtures, the
Dik components can be replaced by the binary diffusivities for the species pairs present.