where il denotes the current density vector. In free electrolyte, there is no source or sink of charge.
where Av denotes the specific surface area (dimension
L2/
L3), and
η the overpotential according to
and Eeq denotes the equilibrium potential for the charge transfer reaction.
where iloc,m (A/m
2) is the
Electrode Reaction current density of the charge transfer electrode reaction of index
m,
il the current density vector in the electrolyte and
is the current density vector in the electrode.
where Av is the specific surface area of the electrocatalyst.
Electron conduction in an Electrode is modeled using Ohm’s law. The domain equation is the following:
where is denotes the current density vector according to:
and where σs denotes the electrical conductivity and
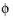 s
s the electrolyte potential.
where il, bnd is a given expression for the current density vector.
The Electrode Current boundary condition sets the total current at a given position in the electrolyte without imposing a current density distribution. The conditions yields a constant electrolyte potential, along the given boundary, that satisfies the total value of the current. The boundary condition is a good choice in the middle of a cell with planar electrodes, where the isopotential level can be a plane (or close to a plane in 3D, or line in 2D) but where the current density distribution is unknown.
The feature adds one unknown variable, the electrolyte potential, 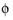 l, bnd
l, bnd, along the boundary. It then adds one additional equation for the total current, which is an integral over the boundary:
The Electrode Current adds one unknown variable, the electric potential,
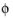 s, bnd
s, bnd, along the boundary. It then adds one additional equation for the total current, which is an integral over the boundary:
and σs denotes the electrode conductivity and
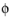 s
s the electric potential. The average current density condition imposes the same equation but multiplies the current density by the area of the boundary to obtain the value of the total current,
In,s.
The Symmetry boundary condition, in the Primary Current Distribution and Secondary Current Distribution interfaces is identical to the Insulation condition and is expressed according to the equation below.
where ik denotes the current density vector and
k =
l,
s is an index for the electrolyte and electrode, respectively.
An applied Electrode Current Density can be defined as its component perpendicular to the boundary according to:
and σs denotes the electrode conductivity and
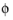 s
s the electric potential.
where is, bnd is a given expression for the current density vector.
where 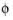 s, ground
s, ground is the ground potential of the cell, and
Ptotal (W) is the power to be drawn.
where Pavg is the average power density on the boundary, and
A is the boundary area.
At the boundary between a liquid electrolyte phase and a ion-exchange membrane phase, denoted l and
m, the Donnan potential,
Δφ(V), describes the relation between the concentration of a species,
cl and
cm (mol/m3), at each side of the boundary and the electrolyte potentials
φl and
φm:
where T(K) is the temperature,
R (mol/(J K)) the molar gas constant,
z the species charge and
F(C/mol) is Faraday's constant.
The current densities Il and
Im in the normal direction
n on each side of the boundary are equal so that the current density over the membrane-liquid interface is continuous:
The molar flux Nl of the charge carrying species in the liquid electrolyte is set proportional to the current density according to Faraday's law: