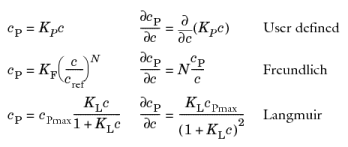Using a Species Source feature, arbitrary expressions can be entered to define, for example, non-equilibrium and temperature-dependent adsorption laws, including those set out by Fetter (
Ref. 7) and Bear and Verruijt (
Ref. 8).
The retardation factor, RF, describes how adsorption slows the solute velocity,
uc, relative to the average linear velocity of the fluid,
ua, as in
If the contaminant moves at the average linear velocity of the fluid for RF =
1. For
RF >
1, the contaminant velocity is smaller than the fluid velocity owing to residence time on solids.